Hypoglycaemia increases the risk of falls, unconsciousness, seizures and dementia in the elderly population. Besides, deepening hypoglycaemia precipitates QT-prolongation, premature cardiac beats and conduction abnormalities.
The old diabetic patient (female, age: 84 ys; HbA1c 7.0%; BMI: 26.8 kg/m2) was admitted to hospital due to recurring episodes of collapse. During the last event of losing consciousness, severe hypoglycaemia was detected (glucose: 1.7 mmol/l). On clinical admission, the electrocardiogram (ECG) recording showed QT-interval prolongation and Mobitz type II 2nd degree AV block being in accordance with findings of a Holter test performed in the preceding weeks.
The decade-long used sulfonylurea (SU) was omitted and the DPP4-inhibitor sitagliptin was added on to metformin as glucose-lowering medication. Neither cardiac conduction abnormalities nor arrhythmias returned during the patient’s hypoglycaemia-free hospital observation and Holter monitoring. Long-lasting severe hypoglycaemia as a side-effect of SU treatment could account for the cardiac arrhythmia.
As for differential diagnosis, carotid ultrasound revealed significant stenosis of the left carotid artery (80-85%) and echocardiographic imaging was negative. Diabetic sensorimotor polyneuropathy and mild-to-moderate cardiovascular autonomic neuropathy was established. However, the role of orthostatic hypotension could be excluded.
Conclusions: Hypoglycaemia may induce cardiac arrhythmias and conduction abnormalities especially in patients with diabetes and cardiac autonomic neuropathy. Administration of glucose-lowering agents with a high risk of hypoglycaemia should be avoided and multifactorial etiology of syncope considered in elderly patients with diabetes.
Keywords: diabetic cardiovascular autonomic neuropathy, hypoglycaemia, arrhythmia
Introduction
Diabetes mellitus is a state of premature death associated with hyperglycaemia [Fisher 2001]. Cardiovascular disease is the major cause of morbidity and mortality in patients with diabetes. Beyond coronary artery disease, the relationship between diabetes and cardiac arrhythmias becomes increasingly apparent as well [Grisanti 2018]. Most arrhythmias are not life-threatening acutely, but prolonged arrhythmic episodes may contribute to the risk of stroke, heart failure and cardiac arrest [Nattel 2014]. The relationship between cardiac arrhythmias and diabetes mellitus might be complex and encompasses components such as glucose control, autonomic nervous system dysfunction, structural remodelling of the heart as well as oxidative stress and inflammation [Grisanti 2018]. As for glycaemic control, studies imply that glucose fluctuations rather than hyperglycaemia contribute to the increased incidence of arrhythmias like atrial fibrillation [Saito 2014]. A body of evidence indicates that hypoglycaemia triggers atrial fibrillation [Celebi 2011, Ko 2018], and severe hypoglycaemic episodes are associated with bradycardia, premature atrial and ventricular beats or even malignant arrhythmias [Stahn 2014, Chow 2014].
Cardiovascular autonomic neuropathy (CAN) is a frequent complication of diabetes with poor prognosis. Silent myocardial infarction and ischaemia are the most widely recognized clinical manifestations associated with its poor prognosis but CAN is also a well-recognized contributor to the occurrence of cardiac arrhythmias [Spallone 2019].
Hypoglycaemia not only contributes to arrhythmias, seizures, disorders of consciousness and traumatic injuries in the acute setting but it is also associated with increased risk of dementia, weight gain as well as impaired quality of life and increased health care expenditures [Amiel 2021]. Moreover, elderly patients with diabetes having many complications and comorbidities take multiple medications that increase the risk of side effects and drug interactions immensely. Hence, treating elderly patients with diabetes might be a challenging clinical scenario.
The authors aim to attract attention to associations between cardiac bradyarrhythmia, cardiovascular autonomic neuropathy and hypoglycaemia with the present case report highlighting specific aspects of antidiabetic treatment in elderly type 2 diabetic patients.
Case report
The 84 year-old patient was admitted to the hospital because of recurring falls and episodes of confusion. Her past medical history included hypertension known for 25 years and type 2 diabetes known for 15 years. As for her medication, she was taking perindopril, amlodipine and indapamide for high blood pressure, and metformin and glimepiride for diabetes.
Recently, the patient has collapsed numerous times and lost consciousness for a short time. The collapses lasted for a few minutes, and based on report of the relatives, the patient turned a little pale in the meanwhile. The patient did not remember actions during fainting, but she did remember the events preceding and following it. During losing consciousness, she did not pass her urine or faeces, and no significant injuries occurred. At the time of the last collapse, the patient was on the way home from holiday by plane. The patient got confused and her speech became incoherent. A stewardess thought of measuring the patient‘s blood sugar and hypoglycaemia was revealed (1.7 mmol/l). The patient was transported from the airport to the emergency department.
In the emergency department, urgent brain CT scan was performed to exclude stroke: it was negative. In emergency care setting, the patient had adequate saturation (SpO2 94%) and her blood pressure was satisfactory (142/75 mmHg). On ECG, sinus rhythm (60/min), some ventricular extrasystoles and left anterior hemiblock, as well as a prolonged QT interval (QTc: 490 ms) were detected. During emergency care, the patient was given i.v. glucose infusion and she was transferred to the department of internal medicine for further observation.
On admission to the ward, she felt dizzy but had otherwise no complaints. She negated adrenergic symptoms hinting to hypoglycaemia before the episode. She admitted that she had experienced dizziness and had fallen several times in the recent months. The patient had also been referred to a cardiologist: a Holter monitoring had been accomplished indicating 2nd degree atrioventricular (AV) block with 2:1 ratio conduction. The patient denied chest pain, shortness of breath or other complaints.
On physical examination, no murmur was heard over the heart, but a systolic murmur could be found over the left carotid artery. The thorax was physically negative. No focal neurological sign was detected. Control ECG showed sinus rhythm (45/min), left anterior hemiblock (BAH) and Mobitz type II second-degree AV block (Figure 1).
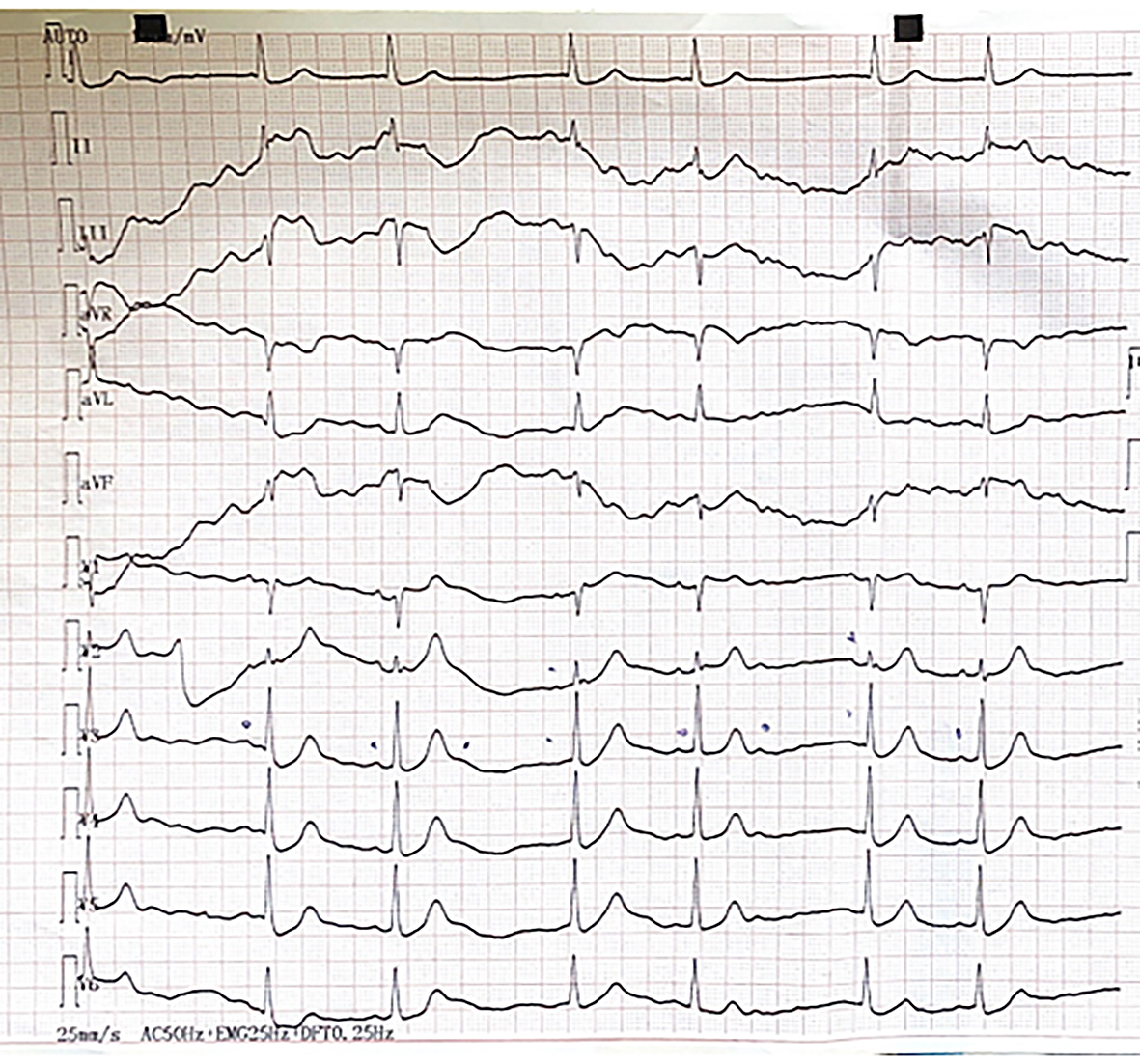 Figure 1: First ECG stripe recorded in the ward shortly after admission as a consequence of hypoglycaemia. It depicts Mobitz type II 2nd degree atrioventricular block. P waves are marked by pen. Not all of the P waves are followed by a QRS complex.
Figure 1: First ECG stripe recorded in the ward shortly after admission as a consequence of hypoglycaemia. It depicts Mobitz type II 2nd degree atrioventricular block. P waves are marked by pen. Not all of the P waves are followed by a QRS complex.
Laboratory tests showed slightly reduced kidney function (urea: 6 mmol/l, creatinine: 126 umol/l), normal electrolytes (Na: 138 mmol/l, K 4.0 mmol/l, Ca: 2.34 mmol/l, P : 1.27 mmol/l) normal blood count (haemoglobin: 145 g/l, white blood cells: 7*109/L, of which neutrophils: 60%, platelets: 313*109/L), mild dyslipidaemia (total cholesterol: 5.6 mmol/l , LDL: 3.6 mmol/l, HDL: 0.8 mmol/l, TG: 1.0 mmol/l), normal liver enzyme values (ASAT: 16 U/l, ALAT: 15 U/l, ALP: 65 U/l, GGT: 10 U/l) and normal blood glucose level (5.1 mmol/l, HbA1c: 6.5%). The uric acid level was 258 µmol/l, the TSH level was 0.460 NE/ml. Chest X-ray depicted sclerotic aortic arch, but otherwise no abnormalities.
The patient‘s blood glucose profile and ECG were closely monitored after admission to the ward. Due to severe hypoglycaemia, the sulfonylurea (glimepiride) treatment was discontinued, while metformin was retained. After that, no hypoglycaemia occurred. Due to higher postprandial blood glucose levels, a DPP4 (dipeptidyl-peptidase) inhibitor (sitagliptin) was added to the metformin treatment.
During the further hypoglycaemia-free monitoring of the patient, the QT interval normalized, no arrhythmias or conduction abnormalities were detected (Figure 2). During Holter monitoring, occasionally atrial or ventricular extrasystoles occurred but no significant pause, AV block, atrial fibrillation or malignant arrhythmia were visible. Although the role of hypoglycaemia in the background of the collapse was clear and all signs pointed to the fact that the arrhythmia was provoked by hypoglycaemia, some further investigations were performed for differential diagnosis.
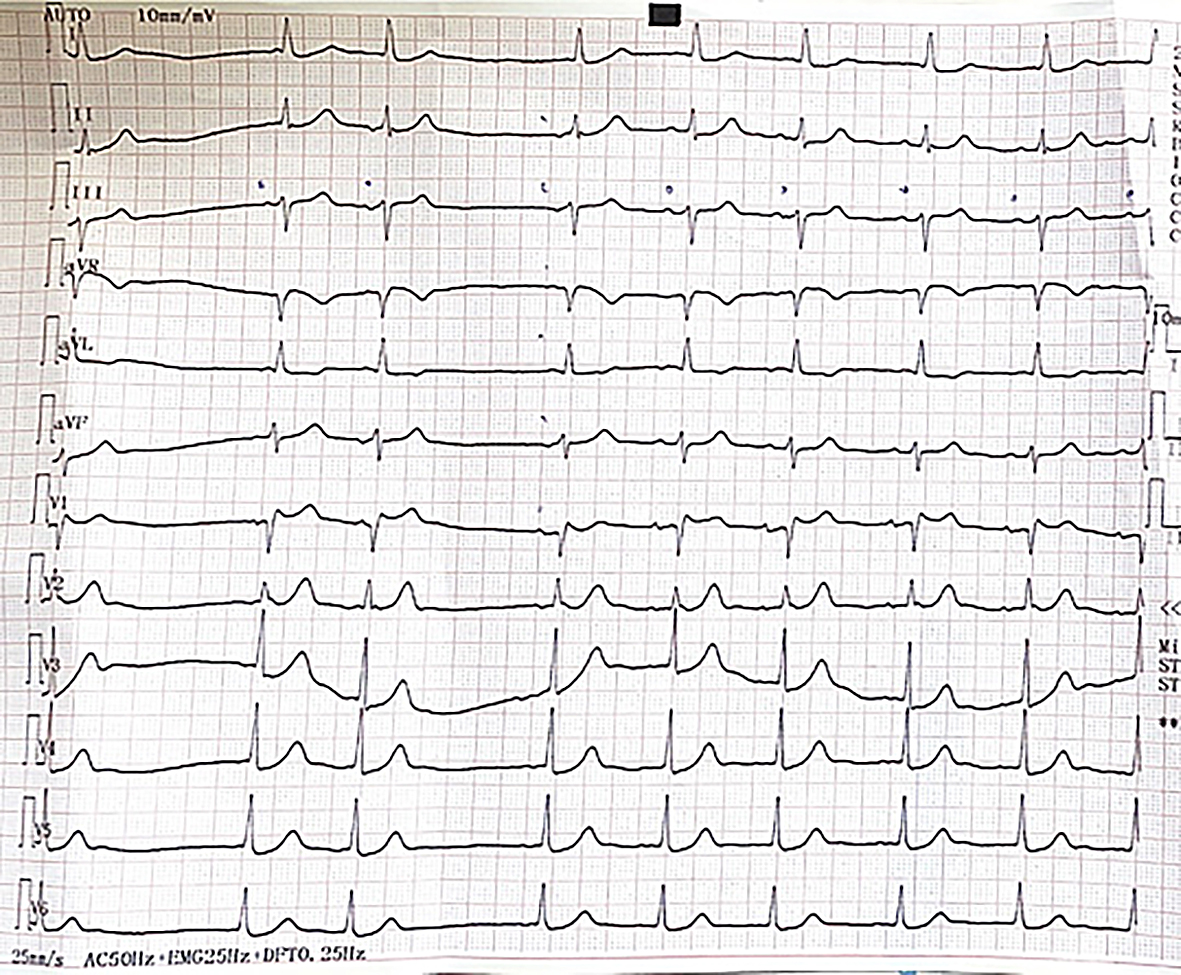 Figure 2: ECG stripe during the hypoglycaemia-free observation of the patient. P waves are marked by pen on the stripe. No arrhythmia or conduction abnomality can be detected except for some supraventricular extrasystoles.
Figure 2: ECG stripe during the hypoglycaemia-free observation of the patient. P waves are marked by pen on the stripe. No arrhythmia or conduction abnomality can be detected except for some supraventricular extrasystoles.
Echocardiography showed preserved left (ejection fraction 55%) and right ventricular function. No wall motion abnormalities were detected. During carotid artery ultrasonography, a 85% narrowing of the left common carotid artery could be verified. Hence, statin and acetyl salicylate therapy were initiated and the patient got an appointment to the vascular surgeon for consultation.
On neuropathy examination, sensory loss of the large fibres of the upper limbs and of all sensory nerve types of both lower limbs was confirmed (Table 1). Sensory function was assessed by the Neurometer (Neurotron Inc., Baltimore, MD, USA) device by measuring current perception thresholds on all sensory nerve fibre types (large myelinated, small myelinated and small unmyelinated nerve fibres). In addition, cardiovascular autonomic function was assessed by using the standard cardiovascular reflex tests (Cardiosys, MDE GmbH, Heidelberg, Germany). Mild-to-moderate cardiovascular autonomic neuropathy was established. No orthostatic hypotension could be diagnosed (Table 2).
Discussion and conclusions
In diabetic patients, especially in the elderly, the pathological role of hypoglycemia should always be sought in the background of loss of consciousness. In this case, hypoglycaemia caused collapses - at least partially - via inducing 2nd degree atrioventricular block. The carotid artery stenosis deteriorated the haemodynamic consequences of heart rhythm and conduction abnormality.
Since a pivotal case series report in 1991, hypoglycaemia has been suggested inducing cardiac arrhythmias and thus being the cause of the so-called ‘dead-in-bed syndrome’ in patients with type 1 diabetes [Tattersal 1991]. In the ORIGIN trial, severe hypoglycaemic episodes were associated with greater risk of all-cause mortality and arrhythmic death [Mellbin 2013]. Gill et al. firstly demonstrated QTc prolongation and cardiac rhythm disturbances in response to nocturnal hypoglycaemic episodes in young patients with type 1 diabetes [Gill 2009]. Later, glucose monitoring in conjunction with simultaneous ECG in type 2 diabetic patients with cardiovascular disease also showed that patients taking insulin/sulfonylurea had a high incidence of severe asymptomatic hypoglycaemia (glucose < 3.1 mmol/l) and had significantly more ventricular arrhythmias compared to those on metformin and DPP-4 inhibitors (7). The association between hypoglycaemia and cardiac arrhythmia has been confirmed in a recent meta-analysis [Li 2023].
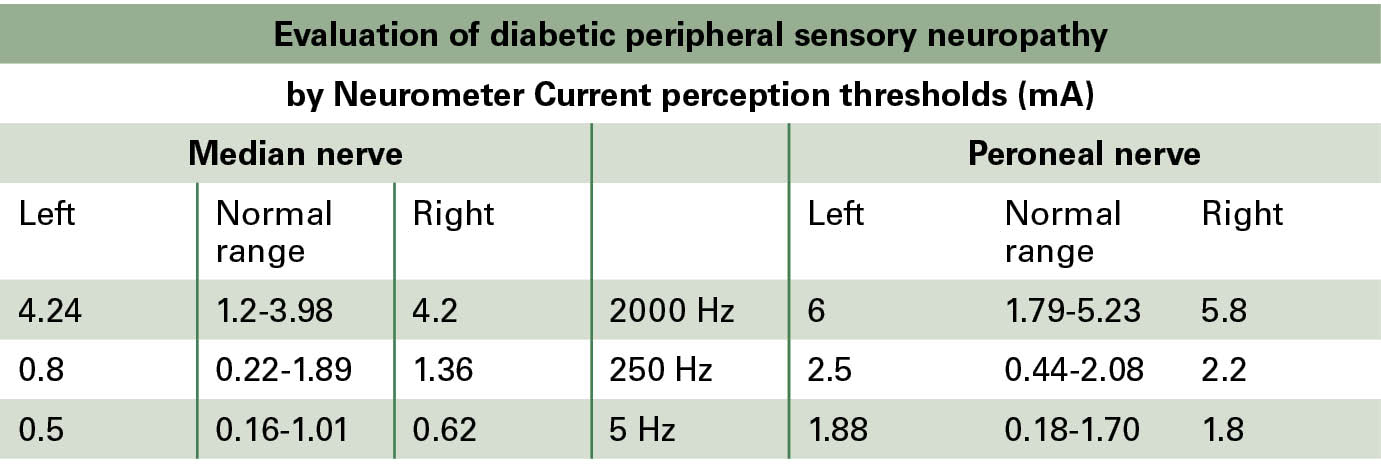 Table 1: Evaluation of the current perception thresholds by the Neurometer device showed diabetic sensorimotor polyneuropathy (hypaesthesia) on the large-fibres of the upper limbs and of all sensory nerve fibre types of both lower limbs.
Table 1: Evaluation of the current perception thresholds by the Neurometer device showed diabetic sensorimotor polyneuropathy (hypaesthesia) on the large-fibres of the upper limbs and of all sensory nerve fibre types of both lower limbs.
The main proarrhythmic effect of hypoglycaemia is QT interval prolongation, but hypoglycaemia is also associated with raised catecholamine levels and lowering of serum potassium augmenting the arrhythmogenic effect of QT prolongation [Gill 2009]. In animal studies, severe hypoglycaemia resulted in a specific pattern of cardiac arrhythmias: it leads to QT prolongation and ventricular extrasystole, ventricular tachycardia, then second and third degree AV block and finally cardiac arrest develop as the blood glucose level gradually decreases [Reno 2013]. QT prolongation is the most consistent finding throughout the studies. Its extent seems to be dependent on numerous factors, including antecedent hypoglycaemia, time spent in hypoglycaemia, diabetes duration and cardiac autonomic neuropathy [Andersen 2020].
The parasympathetic and sympathetic autonomic nervous system is an important regulator of heart rhythm. Cardiovascular autonomic neuropathy is a well-recognized contributor to the occurrence and progression of atrial fibrillation [Agarwal 2017]. Sympathetic overactivity as a consequence of parasympathetic neuropathy leads to increased heart rate that in turn increases the risk of atrial fibrillation and ventricular arrhythmias [Okin 2008, Soliman 2010]. Imbalance in sympathovagal innervation of the heart leading to prolonged QT interval and increased dispersion of repolarization is a major risk factor of ventricular tachycardia and fibrillation as well as sudden cardiac death in patients with diabetes [Rajbhandari 2021, Shimabukuro 1996, Huang 2017]. Hence, our elderly diabetic patient with cardiovascular autonomic neuropathy was particularly prone to ventricular arrhythmia and heart conduction disturbances during hypoglycaemia. Although the association between hypoglycaemia and arrhythmias is getting widely recognised, no one made efforts to reveal the aetiology of heart conduction disturbances shown on prior Holter monitoring in our patient.
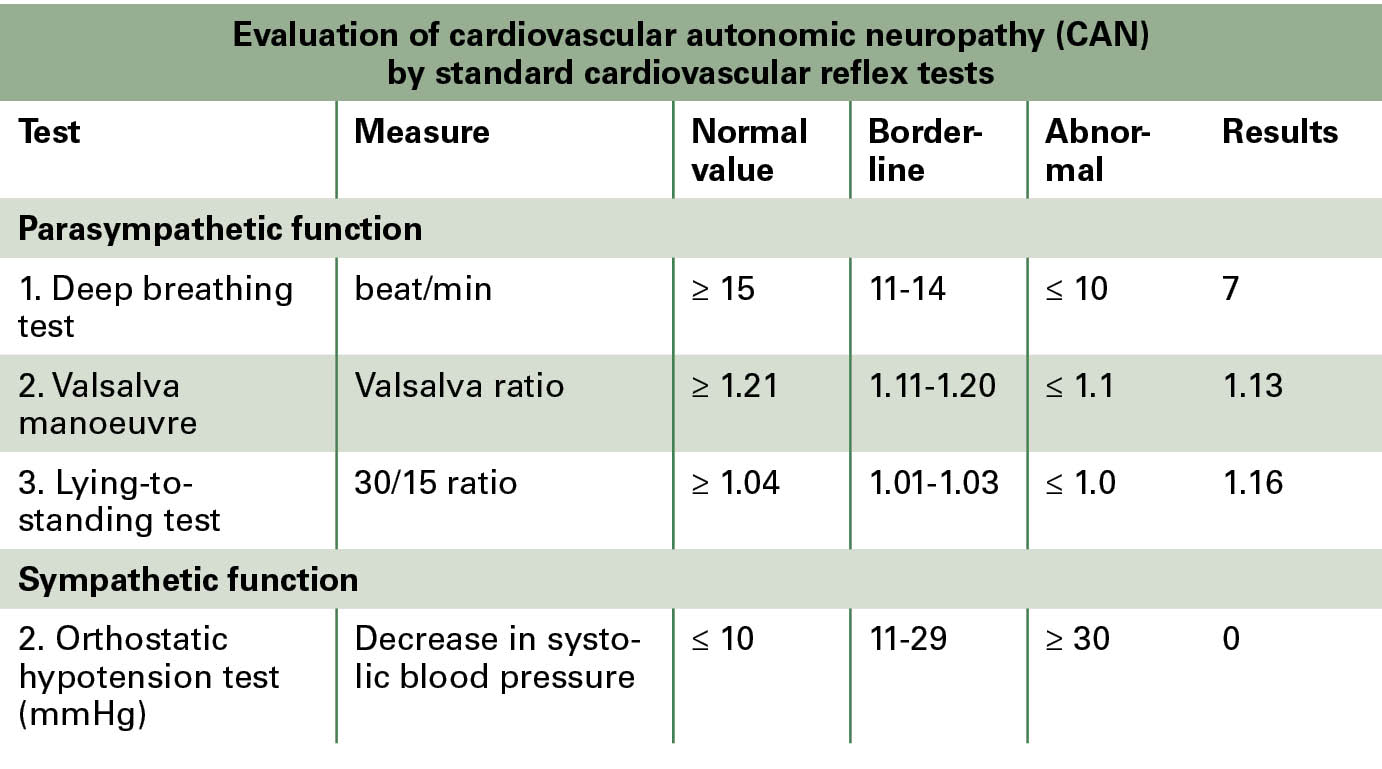 Table 2: Cardiovascular autonomic neuropathy was assessed by the standard cardiovascular reflex tests: mild cardiovascular autonomic neuropathy affecting the parasympathetic nervous system. No orthostatic hypotension could be detected.
Table 2: Cardiovascular autonomic neuropathy was assessed by the standard cardiovascular reflex tests: mild cardiovascular autonomic neuropathy affecting the parasympathetic nervous system. No orthostatic hypotension could be detected.
In elderly diabetic patients, especially those with multiple complications and numerous co-morbidities, the safety and tolerability become the most important aspects of glucose-lowering therapy [American Diabetes Association Professional Practice Committee 2022]. Hypoglycaemia still imposes an inevitable risk in diabetic patients treated with insulin/sulfonylureas and is associated with multiple adverse outcomes. Hypoglycaemic agents are among the top medications associated with emergency unit admissions above the age of 65 years [Budnitz 2011]. In elderly patients, early adrenergic symptoms (anxiety, irritability, diaphoresis, pallor, tachycardia, headache, hunger) of hypoglycaemia are often missing and episodes are only realized when severe and neuroglycopenic symptoms (confusion, slurred speech, blurred vision, seizures, coma) occur. Sympathetic autonomic neuropathy also contributes to this impaired awareness of hypoglycaemia. Consequences of hypoglycaemia are detrimental and reach from impaired quality of life and weight gain to seizures, falls and fractures as well as cardio-thromboembolic events, increased risk and progression of dementia and disability. To avoid hypoglycaemia, the sulfonylurea glimepiride was omitted and DPP4 inhibitor sitagliptin was added on to metformin in our patient. DPP4 inhibitors impose a minimal-to-no risk of hypoglycaemia due to their glucose-dependent effect on glycaemic control. In addition, the risk of side effects or drug interactions is negligible and DPP4 inhibitors represent an extremely safe therapeutic option for the treatment of elderly patients with diabetes [Deacon 2020, Green 2015]. They can be used orally and are also available in fixed combination tablets with metformin at a very reasonable price. These aspects are important as simple therapeutic regimens should be preferred for elderly patients who may suffer from visual or cognitive impairment as well. As for other innovative glucose-lowering medications with minimal-to-no risk of hypoglycaemia, SGLT2 inhibitor or possibly GLP-1 receptor agonist treatment could be considered for our patient. However, the former can only be used with caution in elderly patients, as it can cause volume depletion, hypotension and genitourinary tract infections. A significant barrier to the use of the latter in elderly patients can be the price and the mostly injection formulation. However, it should be emphasised that the choice of glucose-lowering treatment should be individually defined in every patient with respect to their complications, comorbidities, life expectancy, motivation, ability to self-care and socioeconomic status as stated in recent guidelines [American Diabetes Association Professional Practice Committee 2022, Davies 2022].
Those patients still getting sulfonylurea treatment (e.g. in low- and middle-income countries due to limited financial resources), it is very important that they should be educated for symptoms and managing of hypoglycaemia. It is of note, that neither our patient nor her closest relatives were aware of the fact that hypoglycaemia could be the cause of syncope and they did not measure blood glucose at any episode.
In conclusion, the pathological role of hypoglycaemia should always be sought in the background of loss of consciousness in diabetic patients, especially in the elderly. Awareness of hypoglycaemia is reduced with aging, so hypoglycaemia occurs in more severe form. Hypoglycaemia may induce malignant ventricular arrhythmia, conduction block and cardiac arrest. Therefore, glucose-lowering agents with greater risk of hypoglycaemia should be avoided in the elderly, especially in patients with multiple complications, autonomic neuropathy and cardiovascular comorbidities. Furthermore, a multifactorial aetiology of syncope should be considered in elderly patients with diabetes.
|
|
Erschienen in: Diabetes, Stoffwechsel und Herz, 2024; 33 (3) Seite 166-172


