In diabetes mellitus (DM), lipid management is largely identified as one of the four pillars of reducing the risk of diabetes-associated complications, the other being glycaemic and blood pressure control and the use of cardiorenoprotective agents [American Diabetes Association Professional Practice Committee 2025]. The primary goal of lipid management for subjects with risk factors is reduction of low-density lipoprotein cholesterol (LDLc) by ≥ 50 % and maintaining it < 70 mg/dl for all people with DM who have high cardiovascular risk [American Diabetes Association Professional Practice Committee 2025, Marx 2023]. For people with established atherosclerotic cardiovascular disease (ASCVD), even lower LDLc should be achieved (LDLc < 55 mg/dl) [American Diabetes Association Professional Practice Committee 2025, Marx 2023].
Abstract
Suboptimal lipid management is common among people with diabetes mellitus (DM). As tight lipid management is often required, statin therapy is often inadequate to achieve these goals. Proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibition has been of value in this purpose. Recently, inclisiran has been introduced. It has a unique design as a small interfering ribonucleic acid (siRNA) selectively inhibiting PCSK9 messenger ribonucleic acid (mRNA) and thus reducing low-density lipoprotein cholesterol (LDLc). Preliminary analyses of large-scale studies have shown that inclisiran results in a more pronounced LDLc decrease in subjects with DM compared with people with prediabetes or subjects with normoglycaemia. Nevertheless, these preliminary promising outcomes require further validation in clinical studies focused on subjects with DM.
Key words
inclisiran, diabetes mellitus, low-density lipoprotein, PCSK9
Introduction
Statins are widely regarded the standard of care option for lipid management in DM [American Diabetes Association Professional Practice Committee 2025, Marx 2023]. When the treatment goals are not achieved even with the high-intensity statin therapy, the addition of ezetimibe or a proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitor is advisable [American Diabetes Association Professional Practice Committee 2025, Marx 2023]. PCSK9 is an emerging target of dyslipidaemia management, as it selectively increases serum LDLc by reducing available LDL receptors and thus LDL uptake by hepatocytes [Shimada 2015]. The development of therapies targeting PCSK9 has revolutionised pharmacotherapy of dyslipidaemia, resulting in LDLc reduction up to 70 % compared with baseline [Shimada 2015]. The therapeutic efficacy of the monoclonal antibodies evolocumab and alirocumab has been established in large-scale clinical trials [Sabatine 2017, Schwartz 2018]. Favourable outcomes have been reported for people with type 2 diabetes mellitus (T2DM) as well [Rosenson 2019].
The aim of this narrative brief review was to summarise existing evidence on the efficacy of inclisiran in people with DM.
Search strategy
We searched Scopus, PubMed/MEDLINE, and Google Scholar for articles without time restriction, using combinations of the following key words: "inclisiran" and "diabetes mellitus". All types of articles (clinical trials, meta-analyses, case-control studies, observational studies, cross-sectional studies, prospective/retrospective studies, cohort studies, comparative studies, randomised studies, experimental studies) were included. Only articles in English were considered.
DM: diabetes mellitus
HDLc: high density lipoprotein cholesterol
LDLc: low-density lipoprotein cholesterol
mRNA: messenger ribonucleic acid
PCSK9: proprotein convertase subtilisin-kexin type 9
siRNA: small interfering ribonucleic acid
T2DM: type 2 diabetes mellitus
Inclisiran: development
Recently, inclisiran, a new PCSK9 inhibitor with an entirely novel treatment design has been introduced. Inclisiran is a small interfering ribonucleic acid (siRNA) targeting PCSK9 and has been already encompassed in clinical practice guidelines for lipid management [American Diabetes Association Professional Practice Committee 2025, Marx 2023]. Inclisiran has been shown to reduce LDLc in a comparable way as PCSK9-blocking monoclonal antibodies. Nevertheless, this agent has been associated with further benefits: inclisiran not only reduced extracellular, but also intracellular PCSK9 through degrading its messenger ribonucleic acid (mRNA). In addition, inclisiran has a profoundly longer half-time and can be taken only twice a year [Warden 2021]. Furthermore, long-term use of inclisiran has not been linked with any untowards effect or liver toxicity [Wright 2023, LiverTox 2023].
Inclisiran: clinical trials
In one of the first trials assessing the efficacy of inclisiran in 2019, termed ORION-1, significant reductions in LDLc were documented for subjects with inclisiran, both for subjects with DM and for those without (median -28 % to -52 %, p < 0.0001; and -28 % to -55 %, p < 0.005 for all doses in the without- and with-DM groups, respectively) [Leiter 2019]. Moreover, decreased levels of apolipoprotein B, non-high density lipoprotein cholesterol, and lipoprotein(a) and elevated high density lipoprotein cholesterol (HDLc) were documented [Leiter 2019].
In the open-label extension study (ORION-3), conducted between 2017 and 2021, LDLc was reduced by 47.5 %, and 4-year mean reduction was 44.2 % [Ray 2023]. Interestingly, PCSK9 reduction was found to be even more profound ranging from 62.2 % to 77.8 % [Ray 2023]. The safety of inclisiran was confirmed: the incidence of serious adverse effects was 1 % [Ray 2023].
In a recent post-hoc analysis of the ORION-9, ORION-10 and ORION-11 phase 3 randomised trials, individuals with DM or obesity were mainly studied [Leiter 2024]. Participants were stratified according to their glycaemic status in three categories: normoglycaemia, prediabetes and diabetes [Leiter 2024]. LDLc reduction was between 47.6 % and 51.9 % at day 510 compared with baseline [Leiter 2024]. Time-adjusted percentage changes between day 90 and day 540 ranged from 46.8 % to 52.0 % [Leiter 2024]. A significant decrease in PCSK9 and other atherosclerosis-associated lipids and proteins was seen [Leiter 2024]. Inclisiran was efficacious in terms of achieving LDLc targets in people with suboptimal glycaemic control [Leiter 2024]. Mild-to-moderate injection site adverse effects were more frequently reported by inclisiran users compared with controls [Leiter 2024].
Another pooled analysis encompassing data from the ORION-10 and ORION-11 randomised trials included 2975 study participants with established ASCVD, 1182 of whom had DM [Wright 2024]. The benefit of inclisiran was more pronounced in people with DM, with mean percentage difference of 52.2 mg/dl (95 % confidence interval: 48.1 – 56.4 mg/dl), compared to those with normoglycaemia (52.0 mg/dl, 95 % confidence interval: 48.4 – 55.5 mg/dl) or prediabetes (49.6 mg/dl, 95 % confidence interval: 44.1 – 55.2 mg/dl) [Wright 2024].
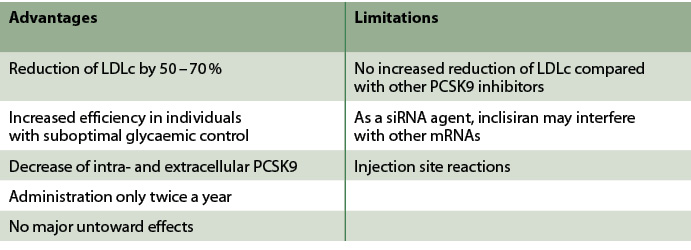 Tab. 1: Advantages and limitations of inclisiran.
Tab. 1: Advantages and limitations of inclisiran.
Discussion
Overall, inclisiran is emerging as a reliable and safe treatment option for lipid management in people with DM. Table 1 provides an overview of advantages and limitations of inclisiran. Data from major large-scale clinical studies have confirmed the efficiency of inclisiran, only administered twice a year, in achieving lipid management targets, as recommended by clinical practice guidelines. Indeed, the recent pooled analysis has indicated that 87.6 % of participants achieved LDLc below 55 mg/dl [Wright 2024]. The safety profile is also satisfactory: only injection site reactions have been reported and no liver-associated adverse outcomes have been found. A potential concern requiring further investigation lies in the specific mechanism of action of inclisiran: as a siRNA, inclisiran could potentially affect other mRNAs beyond PCSK9 mRNA through lower-affinity interactions [Banerjee 2022].
Inclisiran is perhaps the most notable example of novel treatment approaches in lipid management. Recently, the armamentarium of lipid management has been expanded with the introduction of siRNAs targeting lipoprotein(a) and hepatic production of apolipoprotein C-III and circulating triglycerides [Nissen 2025, Watts 2025].
Conclusion
Inclisiran is a very promising agent to revolutionise lipid management, leading to optimal lipid control among people with DM with a reliable safety profile. Nevertheless, additional large-scale clinical studies are required to further validate these promising outcomes among people with DM.
- Inclisiran may be used as an additional LDLc-lowering medication, when optimal lipid control is not achieved with the use of statins in people with DM.
- Apart from injection site reactions, no further notable adverse outcomes have been reported to date.
- Compared with other PCSK9 inhibitors, inclisiran may be administered only twice a year.
|
|
Erschienen in: Diabetes, Stoffwechsel und Herz, 2025; 34 (6) Seite 356-357


